Abstract
Background: Paroxysmal nocturnal hemoglobinuria (PNH) is a rare disease. Parker described three phenotypes: Classic PNH (C-PNH), PNH associated with another bone marrow disorder (BMD-PNH), and subclinical PNH associated with another bone marrow disorder (BMD-SCPNH). Virtually all research of complement inhibitors is focused on C-PNH, while BMD-PNH remains a poorly studied phenotype. The main objective of this study was to analyze survival of BMD-PNH patients compared to that in C-PNH.
Methods: This is a multi-center retrospective study. We analyzed the outcomes of patients diagnosed with PNH from March 1990 to January 2022 in three hospitals in Mexico. PNH was classified according to Parker´s criteria. We compared clinical characteristics between patients with C-PNH and BMD-PNH. Univariate and multivariate analyses were performed to assess the impact of PNH phenotype on overall survival and Kaplan Meier curves were plotted to compare survival between groups.
Results: We found 106 patients diagnosed with PNH, of which 6 patients with the BMD-SCPNH phenotype were excluded. We analyzed 100 patients, median age 49 years (37.5-59), 50% female, C-PNH 72% (n=72), BMD-PNH 28% (n=28). Relevant differences between groups (C-PNH vs BMD-PNH) include serum LDH median 1,147 vs 256 U/L, p=0.001, LDH ≥1.5 SLN 72.2% vs 26.08%, p=0.001, clone size 78.7% vs 18.8%, and platelets median 120x109 U/L vs 13x109U/L, p=0.0001. In the BMD-PNH group, 46.42% (n=13), had a clone size ≥50%, 7.14% (n=2) presented thrombotic events, and 21.42% (n=6) had high disease burden.
Regarding treatment agents (C-PNH vs BMD-PNH), complement inhibitor use was reported in 30.55% (n=22) vs only 3.57% (n=1), p=0.003, and anticoagulation treatment was also more frequent in C-NPH, 47.22% vs 7.14%, p=0.001. Use of bone marrow failure treatment (immunosuppressants and/or androgens) was frequent in the BMD-PNH group, 96.43%, n=27.
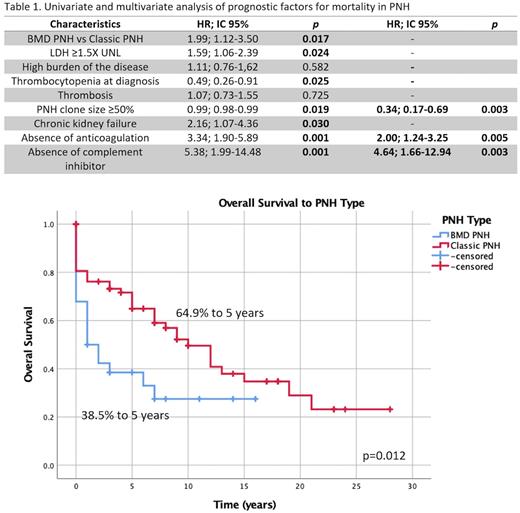
Table 1 describes pronostic factors found to influence survival. Of note, overall survival of BMD-PNH was considerably lower: median 1y (CI95%; 0-2.4h) vs 10y (CI95%;6.6-13.3y), p=0.012, as presented in Fig 1. In the multivariate analysis, lack of either complement inhibitor (HR 4.64; 95% CI, 1.66-12.94, p=0.003) or lack anticoagulation treatment was independently associated with increased mortality (HR 2.00; 95%CI 1.24-3.25, p=0.005).
Conclusions: We found that BMD-PNH patients had a notably inferior survival, and infrequently received agents considered to be standard in C-PNH treatment. Interestingly, 21.42% of BMD-PNH had a high disease burden, a frequent indication of complement inhibitor use in C-PNH. Since specific treatment of BMD-PNH hasn't been defined (that is, beyond immunosuppression and/or androgens), use of complement inhibitor therapy could be a promising option. To our knowledge, clinical trial information of complement inhibitors in BMD-PNH patients is currently lacking.
Disclosures
Alvarez:Novartis,: Speakers Bureau; AbbVie: Speakers Bureau; Bristol: Speakers Bureau; Janssen: Speakers Bureau; Takeda: Speakers Bureau; Teva: Speakers Bureau. Demichelis:Abbvie: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Astellas: Consultancy, Honoraria; Teva: Consultancy; Gilead: Consultancy; ASH: Research Funding; Novartis: Consultancy, Honoraria, Research Funding. Apodaca:Asofarma: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal